thinprep pap test package insert|thinprep pap test instructions : Big box store PreservCyt Solution is designed for use with the ThinPrep Processor, a cytologic preparation device that produces slides for microscopic examination. PreservCyt Solution enables the . Postamos vazados e vídeos "raros" que a maioria das pessoas tem curiosidade em ver. 🗂O que você encontrará aqui; 🆗Vazados 🆗Amador 🆗Packs 🆗Cornos 🆗Flagras 🆗Brigas 🆗Funkeiras 🆗Etc. Open a Channel via Telegram app. Preview channel. Don't have Telegram yet? Open via web telegram. or. Selecionamos os melhores vídeos .
{plog:ftitle_list}
Ontario's Ventilator Equipment Pool Established in 1994, the Ontario Ventilator Equipment Pool (VEP) is a provincial service operated by Kingston Health Sciences Centre that .
thinprep pap test procedure
Download. Hologic is a global champion of women’s health, we integrate The Science of Sure into everything we do to help improve and save lives through early detection and proactive treatment.PreservCyt Solution is designed for use with the ThinPrep Processor, a cytologic .The ThinPrep® 2000 System is intended as a replacement for the conventional .
PreservCyt Solution is designed for use with the ThinPrep Processor, a cytologic preparation device that produces slides for microscopic examination. PreservCyt Solution enables the .
The first liquid-based Pap test with FDA approval/clearance for sample collection of Pap, HPV, chlamydia/gonorrhea, and trichomoniasis testing from the same vial.
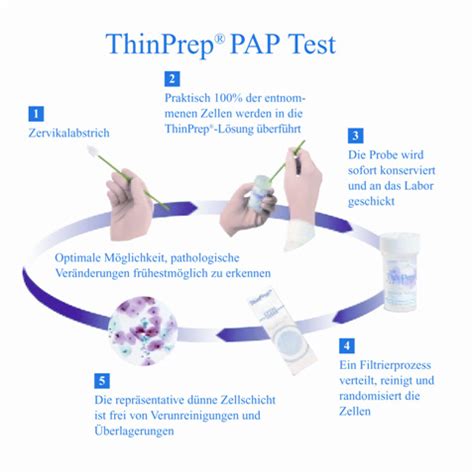
The ThinPrep® 2000 System is intended as a replacement for the conventional method of Pap smear preparation for use in screening for the presence of atypical cells, cervical cancer, or its .The ThinPrep® 3000 Processor (TP-3000) is a device that produces cytologic preparations on glass microscope slides from gynecologic (cervical) samples, and is intended for use in .brush device. Insert the brush into the cervix until only the bottom-most fibers are exposed. Slowly rotate 1/4 or 1/2 turn in one direction. DO NOT OVER-ROTATE. Rinse. .the brush .
Liquid Based Cytology Collection: Quick Reference Guide. ThinPrep® Pap Test Instructions for direct to vial specimen collection. ThinPrep Pap Test Cervical Sampler Broom protocol. .
* The ThinPrep Pap test is the only Pap test with FDA-approved labeling supported by multiple peer-reviewed publications reporting increased glandular disease detection. † Surepath is only .Visit hologic.com/thinprep for more details and best practices for Pap collection. Hologic provides this collection procedure guide as a general informational tool only; it is not an affirmative .
The ThinPrep® 5000 processor is intended as a replacement for the conventional method of Pap smear preparation for use in screening for the presence of atypical cells, cervical cancer, or its .Transfer 1 mL of the fluid remaining in the ThinPrep® Pap test vial into an Aptima® Specimen Transfer tube according to the instructions in the Aptima® Specimen Transfer Kit package insert. Specimens should be transferred to an Aptima® Specimen Transfer tube within 30 days of collection if stored at room temperature (see section 3.2).We offer a multitude of collection devices, including the ThinPrep ® Pap Test Vial, which runs both cervical cancer and STI testing. Our collection devices ensure greater comfort for women and convenience for their providers. . Aptima Multitest Swab [package insert] #AW-14413-001_008, San Diego, CA; Hologic, Inc., 2019 .Note: Refer to the appropriate Aptima package insert for complete storage and handling information. ThinPrep Liquid Cytology Specimens Gynecological specimens may be stored in the ThinPrep liquid cytology vials for at least 30 days at 2°C to 30°C prior to transfer to Aptima Specimen Transfer tubes. Refer to the appropriate Aptima assay .
(Refer to the ThinPrep 2000 System instructions for use for details on performing this step.) 7. Uncap the ThinPrep Pap Test vial, placing the cap on the bench with the threads facing up. Ensure that the bench is clean, with no bleach residue or foreign particles. 8. Load the ThinPrep Pap Test vial into the ThinPrep 2000 System.HPV 16 18/45 genotype assay testing of gynecological specimens collected in ThinPrep Pap Test vials containing PreservCyt solution, and specimens collected in SurePath Preservative Fluid. . package insert for specific shipping and storage conditions. M. Dispose of residual clinical specimens, unused reagents and waste in accordance with local
thinprep pap test pdf
8. Load the ThinPrep Pap Test vial into the ThinPrep 2000 System. From the ThinPrep system main menu, select “4-GYN” by pressing 4 on the keypad. 9. Put on clean gloves. 10. Once the slide preparation is finished, open the door, remove the ThinPrep Pap Test vial and recap the vial. 11. Remove the fixative bath and place the slide in a 95% .Cervical specimens collected in ThinPrep ™ Pap Test vials containing PreservCyt Solution may be tested with the Aptima HPV assay either pre- or post-Pap processing, as well as . transported and stored in accordance with the appropriate package insert, even if theseThinPrep Pap Test Filter during the collection process in order to prevent the cellular . specimens and to the Roche Diagnostics COBAS AMPLICOR CT/NG package insert for instructions for use of that system. If any serious incident occurs related to this device, or any components used with this device, .The ThinPrep 5000 Autoloader system maximizes laboratory efficiency while offering even more walkaway freedom with up to 8 hours of hands-free processing of ThinPrep Pap test, ThinPrep Non-Gyn and ThinPrep UroCyte samples. 52 The ThinPrep 5000 Autoloader system, with continuous access along with chain-of-custody verification, laser etching and automatic vial .
The cobas ® HPV Test for use on the cobas ® 4800 System (cobas ® HPV Test) is a qualitative in vitro test for the detection of Human Papillomavirus in clinician-collected cervical specimens using an endocervical brush/spatula or broom and placed in the ThinPrep ® Pap Test ™ PreservCyt ® Solution or using a cervical broom and placed in SurePath ™ Preservative Fluid.
THINPREP PAP TEST. Η οικογένεια ThinPrep® είναι ο ηγέτης στην πρόληψη του καρκίνου του τραχήλου της μήτρας με σημαντικές πρωτιές: . ThinPrep 2000 System [package insert]. MAN-02060-002 Rev. 001. Marlborough, MA: Hologic, Inc.; 2011. 4. U.S. News & World Report.Transfer 1 mL of the fluid remaining in the ThinPrep® Pap test vial into an APTIMA® Specimen Transfer tube according to the instructions in the APTIMA® Specimen Transfer Kit package insert. Specimens should be transferred to an APTIMA® Specimen Transfer tube within 30 days of collection if stored at room temperature (see section 3.2).The cells are then captured on a gyneco logical ThinPrep Pap test filter that is specifically designed to collect cells. The ThinPrep 5000 processor constantly m onitors the rate of flow through the ThinPrep Pap . Diagnostics COBAS AMPLICOR CT/NG package insert for instructions for use of that system. MAN-04008-001 Rev. 002 Page 4 of 27ThinPrep Pap Test Quick Reference Guide.#DS-05867. Marlborough, MA: Hologic, Inc.; 2017. ACOG Practice Bulletin. 7. . and understand the appropriate package insert and comply with applicable local, state and federal rules and regulations. 3 Rinse .
Transfer 1 mL of the fluid remaining in the ThinPrep® Pap test vial into an APTIMA® Specimen Transfer tube according to the instructions in the APTIMA® Specimen Transfer Kit package insert. Specimens should be transferred to an APTIMA® Specimen Transfer tube within 30 days of collection if stored at room temperature (see section 3.2).Bai H, et al. ThinPrep Pap Test promotes detection of glandular lesions of the endocervix. Diagn Cytopathol. 2000;23:19-22. 10. Carpenter AB, et al. ThinPrep Pap Test: Performance biopsy follow-up in a university hospital. Cancer. .
www.thinprep.com ThinPrep ® Pap Test ™ Quick Reference Guide Endocervical Brush/Spatula Protocol Part No. 85217-001 Rev. H ©2007, Cytyc Corporation 1. Papanicolaou Technique Approved Guidelines (NCCLS Document GP15-A)Contact the cervix with the PAPETTE and insert the central bristles into the cervical canal deep enough to allow the shorter bristles to fully contact the ectocervix. . Rincer la brosse dans la solution PreservCyt® à utiliser avec le ThinPrep® Pap Test™; pour ce faire, pousser la brosse contre le fond du flacon à 10 reprises, en .7. Uncap the ThinPrep Pap Test vial, placing the cap on the bench with the threads facing up. Ensure that the bench is clean, with no bleach residue or foreign particles. 8. Load the ThinPrep Pap Test vial into the ThinPrep 2000 System. From the ThinPrep system main menu, select “4-GYN” by pressing 4 on the keypad. 9. Put on clean gloves.

The ThinPrep® Pap Test with the BD Onclarity™ HPV Assay can be used on the BD COR™ or BD Viper™ LT instrument platforms without the need to change current cytology equipment. The BD COR™ System offers integrated and automated workflows, designed to free up time in the high-throughput laboratory, and the extended claim for the BD .
the ThinPrep Pap test, please follow the instructio ns below. ThinPrep Pap Test Plastic Spatula and Endocervical Brush Protocol . ’ Instruction for Use (IFU) or Package Insert, and it is the medical professionals’ responsibility to read and follow the IFU or Package Insert. The information provided may suggest a particular technique or .
Premarket Approval Application CONFIDENTIAL AND PROPRIETARY Draft Package Insert Print Date: April 4, 2011 Page 1. Roche Molecular Systems, Inc. cobas HPV lest Pleasanton, CA 94588-2722 Labeling . 98 - ThinPrep@ Pap Test" PreservCyt® Solution 99 - .Transfer 1 mL of the fluid remaining in the ThinPrep® Pap test vial into an APTIMA® Specimen Transfer tube according to the instructions in the APTIMA® Specimen Transfer Kit package insert. Specimens should be transferred to an APTIMA® Specimen Transfer tube within 30 days of collection if stored at room temperature (see section 3.2).H. Collect cervical specimens in ThinPrep Pap Test vials containing PreservCyt Solution with broom-type or cytobrush/spatula collection devices according to the manufacturer’s . appropriate package insert, even if these expiration dates have passed. L. Specimens may be infectious. Use Universal Precautions when performing this assay.The CINtec® PLUS Cytology test is a qualitative immunocytochemical assay intended for the simultaneous detection of the p16INK4a and Ki-67 proteins in cervical specimens collected by a clinician using an endocervical brush/spatula or broom collection device and placed in the ThinPrep® Pap Test PreservCyt® Solution. The CINtec PLUS Cytology test
The ThinPrep Pap test has shown to be signiÞcantly more e!ective than conventional Pap testing 1 and has become the preferred . References 1. Table 17, ThinPrep 2000 System [package insert]. MAN-02060-001 Rev. 006, Marlborough, MA: Hologic, Inc.; 2017. Hologic, Inc. 2. Data on File Hologic Report MAN-TPPTBR-001.Transfer 1 mL of the fluid remaining in the ThinPrep® Pap test vial into an APTIMA® Specimen Transfer tube according to the instructions in the APTIMA® Specimen Transfer Kit package insert. Specimens should be transferred to an APTIMA® Specimen Transfer tube within 30 days of collection if stored at room temperature (see section 3.2).
[email protected]. ⚠️ IMPORTANTE: Este e-mail é utilizado unicamente para envios pela Appmax. Precisando de suporte, acesse o artigo Como entrar em .
thinprep pap test package insert|thinprep pap test instructions